Multiple Choice
Given: P4(s) + 6Cl2(g) b 4PCl3(l)
K
Calculate the equilibrium constant for the following reaction.
2PCl3(l) →3Cl2(g) + 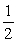 P4(s)
P4(s)
Understand the purpose and effective use of different types of questions in research and interviews.
Comprehend the various types of reports and their specific purposes within a business context.
Recognize the importance and characteristics of planning in business settings.
Identify the characteristics of open and closed questions.
Definitions:
Related Questions
Q3: Calculate the standard enthalpy of formation of
Q30: Consider the following reaction at a certain
Q36: Water and acetone,CH<sub>3</sub>COCH<sub>3</sub>,both freeze at a higher
Q67: What causes the green phosphor?<br>A)ZnS activated with
Q67: An endothermic reaction is most likely
Q69: Calculate the molality of perchloric acid in
Q74: Consider the following cell: Ag(s)|Ag<sup>+</sup>(aq,0.100 M)mAg<sup>+</sup>(aq,0.100 M)|Ag(s)<br>What
Q84: The standard voltage of the cell
Q92: How does an alloy's electrical and thermal
Q100: In solution based methods of nano-material fabrication,what