What is the standard enthalpy of formation of BaCO3(s) ?
BaO(s) + CO2(g) BaCO3(s) ; H° = -269.3 kJ/mol-rxn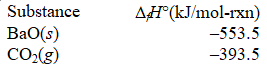
Definitions:
Rockefeller
Refers to John D. Rockefeller, an American industrialist and philanthropist who played a significant role in the early petroleum industry and was considered one of the wealthiest individuals in history.
Kinsey
Refers to Alfred Kinsey, an American biologist and sexologist who founded the Institute for Sex Research at Indiana University and is known for his research on human sexuality.
Living Arrangements
The configuration of a household or living situation, including the individuals who share the space and their relationship to each other.
Children
Young human beings below the age of puberty or below the legal age of majority specified by law.
Q5: Based on electron geometries,which of the following
Q22: At its boiling point of 58.8
Q23: The law of _ states that the
Q24: All of the following statements concerning molecular
Q28: How many values are there for the
Q32: The standard molar enthalpy of formation of
Q36: Which of the following statements is/are CORRECT?<br>1)The
Q37: In which pair do <span
Q42: What mass of Al contains the same
Q49: Which of the following chemical equations