Assume the Reaction Below
2 NO(g)+ O2(g) 2 NO2(g)
Proceeds Via the Following Rate Expression:
Which
Assume the reaction below
2 NO(g) + O2(g) 2 NO2(g)
Proceeds via the following rate expression: 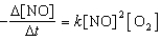
Which of the following statements concerning the above chemical reaction and rate equation is/are CORRECT?
1) The reaction is second-order with respect to NO.
2) The rate of disappearance of O2 is two times the rate of appearance of NO2.
3) According to the balanced chemical equation,the reaction is fifth-order overall.
Definitions:
Small Errors
Minor mistakes or inaccuracies that might occur in various contexts, often not significantly impacting the overall outcome.
Clear Responses
Direct and understandable replies or answers to questions or communications.
Study Team
A group of individuals who come together to share knowledge, resources, and support in pursuit of common academic goals.
Motivator
An internal or external factor that stimulates individuals to act or behave in a certain way towards achieving a goal.
Q11: The density of a gas is 1.96
Q15: What type of colloid is formed when
Q26: Refer to Diagram 9-1.According to molecular orbital
Q28: The equilibrium constant,K<sub>c</sub>,for the decomposition of
Q39: Sodium azide decomposes rapidly to produce
Q45: What is the molar solubility of
Q47: A student analyzed a first-order reaction and
Q50: According to the following cell notation,which species
Q62: The local weather forecaster reports that the
Q78: Given the equilibrium constants for the