The equilibrium constant,Kc,for the decomposition of ammonium hydrogen sulfide is 1.8 10-4 at 25 C.NH4HS(s) 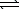 NH3(g) + H2S(g)
NH3(g) + H2S(g)
If excess NH4HS(s) is allowed to equilibrate at 25 C,what is the equilibrium concentration of NH3?
Definitions:
Voice-Training
The process of teaching or adapting speech recognition systems to better understand and respond to a user’s voice or the process of improving one's vocal skills.
Alexa Device
A brand of smart speakers developed by Amazon, designed to respond to voice commands and perform a variety of functions through the Alexa virtual assistant.
Company Logo
A graphic mark, emblem, or symbol used by companies for brand identity and recognition.
Social Media
Platforms and websites where users can create, share, or exchange information, ideas, pictures/videos in virtual communities and networks.
Q9: Nitrogen trifluoride decomposes at to form
Q11: What is the equilibrium pH of an
Q40: In the NO<sub>2</sub><sup>-</sup> ion,each atom can
Q42: Which of the following is true
Q42: For which of the following hypothetical rate
Q43: The pH of a solution at
Q53: If one of the factors determining the
Q68: If an ionic solid has a face-centered
Q76: Which one of the following elements would
Q86: A Faraday,F,is defined as<br>A) the charge on