When gaseous carbon monoxide and hydrogen are combined in a sealed vessel and heated they will eventually form an equilibrium mixture of reactants and products according to the balanced chemical equilibrium below.CO(g) + 3H2(g) 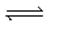 CH4(g) + H2O(g)
CH4(g) + H2O(g)
In one such reaction 3 moles of one reactant were combined with 1 mole of the other reactant in a fixed volume vessel and heated to 1200 K.Analysis of the reaction mixture at various times gave the results below.Which component of the reaction mixture is represented by curve C? 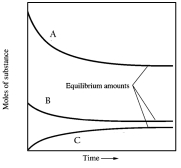
Definitions:
Q6: For the first-order decomposition of N<sub>2</sub>O<sub>5</sub>
Q6: Write a balanced chemical equation for
Q16: What is the volume occupied by
Q23: What is the equilibrium pH of
Q28: Which of the following statements is
Q36: Which of the following substances will have
Q36: Write a net ionic equation for the
Q42: For which of the following hypothetical rate
Q51: What is the highest oxidation state possible
Q60: Which two of the following materials are