What is the reaction quotient,Q,for the equilibrium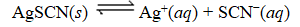 SCN-(aq)
SCN-(aq)
When 0.4257 L of 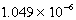
M Ag+ is combined with 0.2376 L of 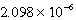
M SCN- in the presence of an excess of AgSCN(s) ?
Definitions:
Behaviors
Actions or reactions of a person or system, often in response to the environment or stimuli.
Misappropriation
The unauthorized use of another's name, likeness, or personal information for personal gain.
Trend
A general direction in which something is developing or changing, often reflected in popular behavior, opinion, or style.
Disclose
The act of making information or data known or public, especially that which was previously hidden or secret.
Q8: For the reaction 2A + B
Q23: For the formation of 1 mol
Q25: Which of the following is the strongest
Q28: Which of the following are valid reasons
Q41: Exactly 1.0 mol N<sub>2</sub>O<sub>4</sub> is placed in
Q53: The pressure exerted by gas molecules inside
Q54: Two important allotropes of phosphorus are
Q67: The following equation is known as _
Q71: Which of the following statements concerning semiconductors
Q86: A certain person has a body temperature