Given the following chemical equilibrium,
COCl2(g) 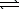
CO(g) + Cl2(g)
Calculate the value of Kc,given that Kp = 6.5 1011 at 298 K.(R = 0.08206 L.atm/mol.K)
Definitions:
Valid Argument
An argument wherein if the premises are true, the conclusion necessarily follows.
Marla's Boat
A possessive phrase indicating that the boat belongs to or is associated with Marla.
Implicit Propositions
Statements or propositions that are not directly expressed but can be inferred from what is explicitly stated.
Syllogistic Steps
The individual stages of reasoning in a syllogism, including the establishment of premises and the deduction of a conclusion.
Q4: How is sodium metal commercially produced?<br>A) By
Q7: One liter of water is mixed
Q9: What is the pH of the
Q13: Below is a proposed mechanism for
Q21: In a first-order reaction,the half-life is
Q27: An aqueous solution contains 0.010 M
Q33: For a reaction,A <span class="ql-formula"
Q33: At 25 <sup> <span class="ql-formula" data-value="\circ"><span
Q65: A 12.0% sucrose solution by mass has
Q69: Polonium (atomic mass 209.0 g/mol)crystallizes in a