Consider the following reaction: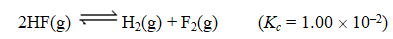
Given that 1.00 mol of HF(g) ,0.389 mol of H2(g) ,and 0.750 mol of F2(g) are mixed in a 5.00-L flask,determine the reaction quotient,Q.
Definitions:
Accumulation
The process of gradually gathering or acquiring an increasing number or quantity of certain items or substances.
Mathematics
The abstract science of numbers, quantity, and space, either as abstract concepts (pure mathematics), or as applied to other disciplines such as physics and engineering (applied mathematics).
Fluid Intelligence
An aptitude for logical thinking and resolving issues in unfamiliar circumstances, without relying on known information.
Notable Achievements
Significant accomplishments or successes recognized for their importance, excellence, or distinction.
Q4: How is sodium metal commercially produced?<br>A) By
Q11: What is the vapor pressure at 20°C
Q17: A possible mechanism for the gas phase
Q29: The conversion (fixation)of N<sub>2</sub> to its compounds
Q33: What is the minimum concentration of
Q40: Given that<br>S(g)+ O<sub>2</sub>(g) <span class="ql-formula" data-value="\to"><span
Q41: What mass of sodium hydroxide must
Q49: Use the following thermodynamic data<br>Species<br><br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg"
Q59: Which of the following statements is/are CORRECT?<br>1)Product
Q90: H<sub>3</sub>PO<sub>3</sub> is a diprotic weak acid.What is