An aqueous mixture of phenol and ammonia has initial concentrations of 0.200 M C6H5OH(aq) and 0.120 M NH3(aq) .At equilibrium,the C6H5O-(aq) concentration is 0.050 M.Calculate K for the reaction.
C6H5OH(aq) + NH3(aq) 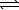 C6H5O- + NH4+(aq)
C6H5O- + NH4+(aq)
Definitions:
Cash Discount
A reduction in the invoice price offered by the seller to encourage early payment by the buyer.
Invoice Date
The date when an invoice is issued, marking the beginning of the grace period for payment.
Partial Payment
A payment made that is not the full amount due but is accepted as a partial settlement of the total debt.
Cash Discount
A reduction in the price of an item or service provided by the seller to the buyer for prompt payment.
Q7: Which of the following liquids has
Q20: Ideally,colligative properties depend only on the<br>A) relative
Q30: What is the molar solubility of
Q32: Which of the following characteristics apply to
Q32: Arrange the three common unit cells in
Q44: A metal crystallizes in a face-centered cubic
Q60: A solution is prepared by dissolving
Q61: The pressure of O<sub>2</sub> in a
Q74: List all the intermolecular forces present in
Q80: The rate constant for a reaction at