Given the following equilibria,
Ni2+(aq) + 2 OH-(aq) 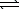 Ni(OH) 2(s) K1 = 1.8 1015
Ni(OH) 2(s) K1 = 1.8 1015
Ni2+(aq) + 4 CN-(aq) 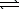 Ni(CN) 42-(aq) K2 = 2.0 1031
Ni(CN) 42-(aq) K2 = 2.0 1031
Determine the equilibrium constant,Kc,for the following reaction.
Ni(OH) 2(s) + 4 CN-(aq) 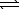 Ni(CN) 42-(aq) + 2 OH-(aq)
Ni(CN) 42-(aq) + 2 OH-(aq)
Definitions:
Schachter Theory
A psychological theory suggesting that emotions are determined by physiological arousal and its cognitive interpretation.
Emotional States
The internal conditions that arise from feelings and emotions, affecting mood and behavioral responses.
Physiological Cues
Bodily signals that indicate internal states or responses to external stimuli.
Parenthood
The state or process of being a parent and the responsibilities associated with it.
Q5: How many moles of HCl must be
Q16: Consider the following half-reactions:<br>Cl<sub>2</sub>(g)+ 2 e<sup>-</sup>
Q19: Nitrogen dioxide reacts with carbon monoxide
Q22: Which theory,valence bond or molecule orbital,correctly predicts
Q29: Write a balanced chemical equation for
Q33: Balance the following half-reaction occurring in
Q36: You have a 30.0 L cylinder
Q46: Which of the following is
Q56: Which of the following concerning the
Q81: Gold and platinum are commonly used as