Assume that the following chemical reaction is at equilibrium.
2 ICl(g) 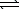 I2(g) + Cl2(g) H = +26.9 kJ
I2(g) + Cl2(g) H = +26.9 kJ
At 25 C,Kp = 2.0 105.If the temperature is increase to 45 C,which statement applies?
Definitions:
Movement-related Side Effects
Undesirable physical effects primarily associated with taking certain medications, leading to involuntary movements or altered muscle control.
Hallucination And Delusions
Hallucinations refer to false sensory experiences, while delusions involve firmly held false beliefs, both of which are common symptoms in various psychiatric disorders.
Antipsychotic Drugs
Medications primarily used to manage psychosis, including delusions, hallucinations, paranoia, or disordered thought, associated with various mental health conditions.
Tardive Dyskinesia
A neurological disorder characterized by involuntary, repetitive body movements, often as a side effect of long-term antipsychotic medication use.
Q9: As pure molecular solids,which of the following
Q19: A 0.10 M solution of a
Q21: A physics experiment is conducted at a
Q34: A gas sample is heated from -20.0°C
Q43: At what temperatures will a reaction
Q43: What is the reaction quotient,Q,for the equilibrium<br><img
Q45: What is the correct name for NO<sub>3</sub><sup>-</sup>?<br>A)
Q49: Which of the following do
Q61: Which one of the following sets of
Q72: What is the equilibrium constant (K)at 25°C