When gaseous carbon monoxide and hydrogen are combined in a sealed vessel and heated they will eventually form an equilibrium mixture of reactants and products according to the balanced chemical equilibrium below.CO(g) + 3H2(g) 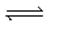 CH4(g) + H2O(g)
CH4(g) + H2O(g)
In one such reaction 3 moles of one reactant were combined with 1 mole of the other reactant in a fixed volume vessel and heated to 1200 K.Analysis of the reaction mixture at various times gave the results below.Which component of the reaction mixture is represented by curve C? 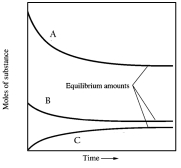
Definitions:
Black-Scholes Model
A mathematical model used for pricing options, providing estimates of the variations over time of financial instruments.
European Put Options
A type of put option that can only be exercised at the expiration date, allowing the holder to sell the underlying asset at a specified strike price.
American Put Options
Financial instruments that give the holder the right to sell a specified amount of an underlying asset at a set price before the option expires.
Call Option
A financial contract giving the buyer the right, but not the obligation, to purchase an asset or security at a predetermined price within a specific time frame.
Q6: The Henry's law constant for O<sub>2</sub>
Q15: What is the H<sub>3</sub>O<sup>+</sup> concentration in
Q21: A salt with a 1:1 ratio of
Q35: Which of the following strong electrolytes has
Q41: Exactly 1.0 mol N<sub>2</sub>O<sub>4</sub> is placed in
Q43: At what temperatures will a reaction
Q55: Methane gas,CH<sub>4</sub>,effuses through a barrier at a
Q64: At 25 <sup> <span class="ql-formula" data-value="\circ"><span
Q77: What is the mole fraction of urea,CO(NH<sub>2</sub>)<sub>2</sub>,<sub>
Q84: When a secondary battery provides electrical energy,it