Write a balanced chemical equation which corresponds to the following equilibrium constant expression. 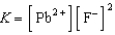
Definitions:
Q5: Given the following,determine <span class="ql-formula"
Q10: A sample of methane,CH<sub>4</sub>,occupies a volume of
Q14: Which equation depicts aqueous hydrogen sulfide behaving
Q15: A 10.0-g sample of solid NH<sub>4</sub>Cl is
Q20: Which ion occurs in the highest concentration
Q41: <span class="ql-formula" data-value="\Delta"><span class="katex"><span class="katex-mathml"><math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow><mi mathvariant="normal">Δ</mi></mrow><annotation
Q44: Real gases are those that<br>A) only behave
Q66: Given the initial rate data for
Q69: The chemical equations below show the reaction
Q82: The hydroxide ion concentration of a saturated