The chemical equations below show the reaction of Al(OH)3 as a Lewis acid and as a Lewis base,respectively.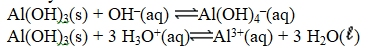
Substances that can behave as either Lewis acids or bases are called ________ substances.
Definitions:
Double-Declining-Balance
A method of accelerated depreciation that applies a constant rate of depreciation to a declining book value, resulting in higher depreciation expense in the early years of the asset's life.
Straight-Line
A method of depreciation that allocates the cost of an asset evenly over its useful life, reflecting a constant rate of expense over each period.
Depreciation Methods
Various approaches to allocating the cost of an asset over its useful life, such as straight-line, declining balance, and units of production methods.
Units-Of-Production
A depreciation method that allocates an asset's cost based on its usage, output, or production, rather than mere passage of time.
Q2: At a high temperature,equal concentrations of 0.160
Q16: At 37°C,what is the osmotic pressure
Q18: Using the thermodynamic data below,and a value
Q30: Which of the following nonpolar molecules has
Q31: Which of the following pairs of
Q58: Which of the following conclusions concerning the
Q59: Which of the following is the correct
Q66: On a relative basis,the weaker the intermolecular
Q68: Which of the following compounds has a
Q74: A 1.0-liter solution contains 0.25 M