Given the following chemical equilibrium,
COCl2(g) 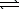
CO(g) + Cl2(g)
Calculate the value of Kc,given that Kp = 6.5 1011 at 298 K.(R = 0.08206 L.atm/mol.K)
Definitions:
Binding
A legal agreement or practice that is enforceable in a court of law.
Private Law
Law that involves suits between private individuals or groups.
Private Individuals
Persons not acting in any official capacity or representing any entity or the government, conducting affairs for personal or non-public interests.
Market Transactions
Exchanges or trades of goods, services, or financial instruments between parties in a marketplace.
Q8: For a certain reversible process q
Q11: In general,as temperature increases,the rate of a
Q34: Henry's law states that the solubility of
Q40: The elements of group 5A,the nitrogen family,form
Q42: Which of the following is not a
Q48: Use your knowledge of group properties to
Q53: You have 75.0 mL of 0.11
Q56: The change in entropy for any process
Q69: If 0.357 g of CH<sub>4</sub> gas
Q84: What color change is exhibited by phenolphthalein