At a high temperature,equal concentrations of 0.160 mol/L of H2(g) and I2(g) are initially present in a flask.The H2 and I2 react according to the balanced equation below.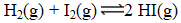
When equilibrium is reached,the concentration of H2(g) has decreased to 0.036 mol/L.What is the equilibrium constant,Kc,for the reaction?
Definitions:
Holiday Party
A social gathering organized to celebrate a holiday or festive season, often involving recreational activities, food, and entertainment.
Administrative Expense
Expenses that are not directly tied to the production or selling of goods and services, such as salaries of senior executives and costs of general services.
Period Cost
Costs that are not directly tied to the production process and are expensed in the period they are incurred, such as selling and administrative expenses.
FIFO Cost Flow
An inventory valuation method where the oldest inventory items are recorded as sold first, leaving the newest items in inventory.
Q11: The electron configuration of a particular
Q15: What type of colloid is formed when
Q21: Consider the following reaction:<br>2C(s)+ 2H<sub>2</sub>(g) <span
Q29: Which of the following concerning
Q29: In what way is cryolite (Na<sub>3</sub>AlF<sub>6</sub>)used in
Q37: What does the following figure represent?
Q51: The standard enthalpy of formation of RbF(s)is
Q62: The standard enthalpy of formation of ammonia
Q78: A buffer contains 0.50 mol NH<sub>4</sub><sup>+</sup> and
Q85: Which of the following substances is never