At 25 C,0.138 mg AgBr dissolves in 10.0 L of water.What is the equilibrium constant for the reaction below?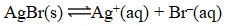
Definitions:
Average Cost
The total cost divided by the number of units, used to calculate cost of goods sold and ending inventory valuation.
Work in Process
Inventory that includes materials that are currently being processed into finished goods but are not yet complete.
Standard Costs
Predetermined costs for materials, labor, and overhead intended as targets for performance evaluation.
Kilos
A unit of measure equivalent to 1000 grams or approximately 2.20462 pounds, often used for measuring weight.
Q9: What is the pH of the
Q16: Which of the following statements are CORRECT?<br>1)Chili
Q20: When mixed in appropriate amounts,each of the
Q32: Which of the following statements concerning a
Q33: Balance the following half-reaction occurring in
Q37: What volume does 40.5 g of N<sub>2</sub>
Q50: If 500 mL of 1.2
Q59: All of the following elements or compounds
Q62: The local weather forecaster reports that the
Q71: A 2.50-mol sample of HI is