Multiple Choice
Assume that the following chemical reaction is at equilibrium.
2 ICl(g) 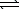 I2(g) + Cl2(g) H = +26.9 kJ
I2(g) + Cl2(g) H = +26.9 kJ
At 25 C,Kp = 2.0 105.If the temperature is increase to 45 C,which statement applies?
Definitions:
Related Questions
Q24: As defined by Ludwig Boltzmann,the third law
Q33: What is the minimum concentration of
Q37: What volume does 40.5 g of N<sub>2</sub>
Q39: Which of the following is the second
Q50: What is the hydroxide-ion concentration of
Q51: Which of the following expressions is not
Q59: When a glass tube with a small
Q61: For the hypothetical reaction aA
Q69: If 0.357 g of CH<sub>4</sub> gas
Q82: In the following reaction,<br>HCO<sub>3</sub><sup>-</sup>(aq)+ H<sub>2</sub>O( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg"