Given that Ka for the weak acid HA is 3.46 10-8,calculate K for the reaction of HA with OH-.HA(aq) + OH-(aq) . A-(aq) + H2O( 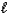 )
)
Definitions:
1-bromopropane
An organobromide compound used as a solvent or an intermediate in organic synthesis, featuring a three-carbon chain.
Synthetic Steps
The individual chemical reactions or stages used to manufacture a compound in the laboratory or industry.
Major Organic Product
The core product that is created in the highest quantity during an organic chemical operation.
Reaction
A process in which substances interact to form one or more new substances characterized by a change in their chemical properties or composition.
Q6: Hyperventilation can cause your blood pH to
Q18: Which of the following properties of water
Q21: Consider the following reaction:<br>2C(s)+ 2H<sub>2</sub>(g) <span
Q25: Which of the following might be used
Q32: How many mechanistic steps are depicted by
Q42: Which of the following conditions is/are met
Q44: Which statement concerning relative rates of
Q48: Carbon monoxide reacts with oxygen to
Q57: Which of the following statements is/are CORRECT?<br>1)Solubility
Q75: What is the total number of valence