Given the following acid dissociation constants,
Ka (H3PO4) = 7.5 10-3
Ka (NH4+) = 5.6 10-10
Determine the equilibrium constant for the reaction below at 25 C.H3PO4(aq) + NH3(aq) 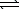 NH4+(aq) + H2PO4-(aq)
NH4+(aq) + H2PO4-(aq)
Definitions:
Reminisce
The act or practice of recalling and sharing memories from the past, often used therapeutically in elder care to improve mood and cognitive function.
Communication Technique
Methods or strategies used to convey information, ideas, or feelings effectively between individuals or groups.
AIDET
An acronym that stands for Acknowledge, Introduce, Duration, Explanation, and Thank you, used to guide healthcare providers in communicating with patients to improve their experience.
Describes Procedures
The detailed explanation or documentation of how specific tasks or operations should be carried out.
Q6: Nitric acid decomposes slowly in sunlight
Q9: What compound was used by the ancient
Q12: What process is used to covert coal
Q14: Calcium crystallizes in a face-centered cubic lattice.The
Q38: Identify the following orbital. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg" alt="Identify
Q44: What is the OH<sup>-</sup> concentration of
Q45: Which acid-base reaction results in an
Q68: If an ionic solid has a face-centered
Q70: A 3.00-liter flask initially contains 3.00
Q81: The solubility of copper(II)oxalate is 3.2