Given that Ka for the weak acid HA is 3.46 10-8,calculate K for the reaction of HA with OH-.HA(aq) + OH-(aq) . A-(aq) + H2O( 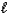 )
)
Definitions:
Vote
The act of making a choice in an election or decision-making process.
Financing Corporation
A company that provides money to individuals or businesses for the purpose of investment or purchase, expecting repayment usually with interest.
Advantages and Disadvantages
This term refers to the positive and negative aspects of a specific situation, decision, or strategy.
Legal Myth
A belief about the law or legal system that is not true or is based on incorrect assumptions.
Q2: What is the hydronium-ion concentration in
Q4: How is sodium metal commercially produced?<br>A) By
Q6: If a chemical reaction is exothermic,but
Q15: Diluting concentrated sulfuric acid with water
Q21: Calculate the equilibrium constant for the
Q40: Which process requires the greatest endothermic change
Q42: An unknown white solid was found to
Q42: A surfactant used for cleaning is called
Q65: Strontium oxide has a face centered
Q77: Write the expression for K for the