Given the following acid dissociation constants,
Ka (H3PO4) = 7.5 10-3
Ka (NH4+) = 5.6 10-10
Determine the equilibrium constant for the reaction below at 25 C.H3PO4(aq) + NH3(aq) 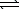 NH4+(aq) + H2PO4-(aq)
NH4+(aq) + H2PO4-(aq)
Definitions:
Problem-solving Mentality
An approach or mindset focused on identifying solutions to problems or overcoming obstacles effectively.
Restraining Forces
Factors that resist or counteract change, preventing movement towards a desired state or objective.
Resistance Change
The opposition or pushback by individuals or groups within an organization against changes in processes, systems, or culture.
Process Forces
The dynamic elements within group interactions that contribute to the method of how tasks are approached, such as communication and decision-making protocols.
Q11: What is the pH of a
Q11: The largest known source of tar sands
Q17: Aluminum is resistant to corrosion because<br>A) the
Q27: Which of the following linear chain alcohols
Q48: Use your knowledge of group properties to
Q55: What is the pH of an
Q58: As pure molecular solids,which of the following
Q58: What is the pH of the
Q58: The calcium silicate formed in the blast
Q59: The elementary steps for the catalyzed