Multiple Choice
The complete reaction of an acid and base is as follows.HClO4(aq) + LiOH(aq) H2O( 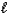 ) + LiClO4(aq)
) + LiClO4(aq)
What is the equilibrium constant for the net ionic reaction at 25 C?
Definitions:
Related Questions
Q4: Which of the following statements concerning real
Q4: How is sodium metal commercially produced?<br>A) By
Q15: The solubility of barium carbonate (BaCO<sub>3</sub>)in water
Q22: Which of the following occurs uncombined in
Q31: Potassium crystallizes in the body-centered cubic system.If
Q41: Exactly 1.0 mol N<sub>2</sub>O<sub>4</sub> is placed in
Q61: For the hypothetical reaction aA
Q66: The product of the oxidation of iron
Q73: Which list contains the three most abundant
Q76: What is the OH<sup>-</sup> concentration in