Calculate the molar concentration of uncomplexed Zn2+(aq) in a solution that contains 0.24 M Zn(NH3) 42+ and 0.3671 M NH3 at equilibrium.Kf for Zn(NH3) 42+ is 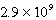 .
.
Definitions:
Court of Law
An official agency empowered to adjudicate conflicts between entities and dispense justice in civil, criminal, and administrative cases.
Statutory Law
The body of law derived from statutes enacted by legislative bodies.
International Law
A body of rules and principles that governs the relations and conduct of nations and international organizations.
Paralegals
Professionals trained in subsidiary legal matters who work under the supervision of lawyers to assist in various legal tasks.
Q1: All of the following statements concerning ligand
Q12: An SHE electrode has been assigned
Q18: Which of the following alcohols is likely
Q27: When an aqueous solution of sodium
Q40: Consider the following equilibrium:<br>CO<sub>2</sub>(g)+ H<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg"
Q51: The addition of Br<sub>2</sub> is used as
Q54: What is the balanced spontaneous reaction
Q57: Which of the following statements is/are CORRECT?<br>1)Solubility
Q58: Which of the statements concerning the phase
Q83: The concentration of Pb<sup>2+</sup> in an