Calculate the molar concentration of uncomplexed Zn2+(aq) in a solution that contains 0.24 M Zn(NH3) 42+ and 0.3671 M NH3 at equilibrium.Kf for Zn(NH3) 42+ is 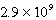 .
.
Definitions:
Entry Of Firms
The process where new businesses enter a market to compete with existing firms, contributing to innovation, competition, and potentially altering market dynamics.
Monopolistically Competitive
Monopolistically competitive refers to a market structure characterized by many firms selling similar but not identical products, allowing for some degree of market power and product differentiation.
Price
Price refers to the sum of money that is anticipated, demanded, or paid in exchange for something.
Monopolistically Competitive
A market configuration where numerous companies offer goods that are alike but not the same, granting them a measure of control over the market.
Q7: Why do salts containing basic anions have
Q16: Silver chloride crystallizes with the sodium chloride
Q34: Henry's law states that the solubility of
Q36: Which group 1A metal is the strongest
Q40: What is the coordination number of the
Q48: A solution in which there is more
Q54: What is <span class="ql-formula" data-value="\Delta"><span
Q57: Which of the following statements is/are CORRECT?<br>1)Solubility
Q58: A reaction is product-favored when<br>A) Q <
Q71: A 2.50-mol sample of HI is