For the reaction given below, H0 = -1516 kJ at 25 C and S0 = -432.8 J/K at 25 C.This reaction is spontaneous ____.
SiH4(g) + 2O2(g) SiO2(s) + 2H2O 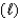
Definitions:
Delivery
The process of transferring an item from the seller to the buyer, or the act of fulfilling a contractual obligation or transferring ownership.
Payment Obligation
A legally binding commitment to pay a specified amount of money to another party under the terms of a contract or agreement.
Impossibility
A legal defense in contracts referring to situations where an unforeseen event makes it impossible for a party to fulfill their contractual obligations.
Remote Event
An occurrence that has a very low probability of happening, often considered insignificant in decision-making processes due to its unlikely nature.
Q10: The minimum wavelength of light required
Q12: If the concentration of sodium carbonate
Q18: An aqueous solution with a pH of
Q32: How many mechanistic steps are depicted by
Q37: How many moles of solid NaF
Q43: Which relationship correctly compares the rates
Q44: All of the following molecules or ions
Q48: A solution in which there is more
Q60: Which of the following statements is/are
Q83: Which of the following species is the