The standard free energy change associated with the dissolution of ammonium nitrate in water is -6.73 kJ/mol at 298.15 K.
NH4NO3(s) 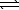 NH4NO3(aq)
NH4NO3(aq)
What is the equilibrium constant for the reaction? (R = 8.314 J/K.mol)
Definitions:
Unit Variable Cost
The cost associated with producing one additional unit of a product, which includes materials, labor, and other variable costs.
Break-even Chart
A graphical representation showing the point at which total costs and total revenue are equal, thus indicating no profit or loss.
Fixed Costs
Business expenses that remain constant regardless of the level of production or sales, such as rent, salaries, and insurance premiums.
Variable Costs
Expenses that change in proportion to the level of production or sales volume, such as raw materials or direct labor costs.
Q6: Write a balanced chemical equation for
Q11: Al<sup>3+</sup> is reduced to Al(s)at an
Q19: Which of the following statements concerning
Q25: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q25: Catalytic steam reformation of hydrocarbons (such as
Q28: What is the hydronium-ion concentration of
Q53: Given that K<sub>a</sub> for the weak
Q62: Which of the following reactions would
Q69: Write the expression for K<sub>p</sub> for the
Q78: Given the equilibrium constants for the