For the reaction given below, H0 = -1516 kJ at 25 C and S0 = -432.8 J/K at 25 C.This reaction is spontaneous ____.
SiH4(g) + 2O2(g) SiO2(s) + 2H2O 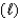
Definitions:
Social Dilemmas
Situations in which individual decision-makers face choices where the outcome of one choice will lead to suboptimal outcomes for all involved parties.
Fossil Fuels
Natural fuels such as coal, oil, and natural gas formed from the decomposed remains of ancient plants and animals, used as a primary energy source.
Tragedy of the Commons
A situation in a shared-resource system where individual users acting independently according to their own self-interest behave contrary to the common good of all users by depleting or spoiling that resource.
Public Goods Dilemma
A situation in which the consumption of a good by one individual does not reduce its availability to others, leading to potential overuse or depletion.
Q6: Hyperventilation can cause your blood pH to
Q23: What is the molar solubility of
Q23: The period 6 transition metals have greater
Q24: Suppose 50.00 mL of 2.0
Q26: For the reaction given below,2.00 moles of
Q28: Which of the following is not a
Q45: In an experiment,0.42 mol H<sub>2</sub> and 0.42
Q50: If 500 mL of 1.2
Q52: Which of the following statements concerning solubility
Q67: The rate constant for a first-order