If rG for the following reaction is -22.2 kJ/mol-rxn,calculate 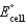 .
.
Cu2+(aq) + 2 Ag(s) + 2 Cl-(aq) Cu(s) + 2 AgCl(s)
Definitions:
Genetic Predispositions
Natural tendencies or susceptibilities influenced by genetic makeup that may increase the chance of developing particular traits or disorders.
Sandwich Generation
A generation of people who care for their aging parents while supporting their own children.
Middle Adulthood
A stage of life, typically considered to be from about 45 to 65 years of age, characterized by stability, sound relationships, and a sense of contributing to society.
Adolescence
The transitional stage of physical and psychological development that generally occurs during the period from puberty to legal adulthood.
Q2: At a high temperature,equal concentrations of 0.160
Q12: What is the number of unpaired electrons
Q19: A certain radioactive isotope has a
Q25: What is the minimum mass of
Q29: For which of the following substances is
Q34: What is the H<sub>3</sub>O<sup>+</sup> concentration in
Q44: What is the value of the
Q47: Metal hydrides are used to remove
Q66: Courts can rely on the common law
Q70: All of the following statements concerning acid-base