The following has a potential of 0.92 V: 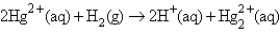 If the concentrations of the ions were 1.0 M and the pressure of H2 were 1.0 atm,then E for the half-reaction
If the concentrations of the ions were 1.0 M and the pressure of H2 were 1.0 atm,then E for the half-reaction 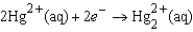
Would be
Definitions:
Since
A word used to indicate time or causality in a statement or argument.
Communication Skills
The abilities used in effective conveying, sharing, or exchanging of information and ideas with others.
Identifying
The process of recognizing and naming someone or something based on their characteristics or attributes.
Issue
An ill-defined complex of problems involving a controversy or uncertainty.
Q4: The _ of an elementary step is
Q8: For a certain reversible process q
Q14: Which of the following statements is/are CORRECT?<br>1)Spontaneous
Q27: Which of the following noncyclic hydrocarbons
Q41: What is the classification of the molecule
Q53: The flexibility,strength,hardness,and malleability of carbon steel is
Q53: Calculate the standard entropy change for the
Q67: Given the following chemical equilibrium,<br>COCl<sub>2</sub>(g) <img
Q71: What is the boiling-point <span
Q71: Which element has the following ground