Calculate the equilibrium constant for the following reaction at 25 C,
2 IO3-(aq) + 5 Hg( 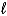 ) + 12 H+(aq) I2(s) + 5 Hg2+(aq) + 6 H2O(
) + 12 H+(aq) I2(s) + 5 Hg2+(aq) + 6 H2O( 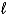 )
)
Given the following thermodynamic information.
IO3-(aq) + 6 H+(aq) + 5 e- I2(s) + 3 H2O( 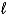 ) E = +1.20 V
) E = +1.20 V
Hg2+(aq) + 2 e- Hg( 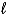 ) E = +0.86 V
) E = +0.86 V
Definitions:
Problem Solving
The process of recognizing, defining, and solving a problem.
Sociological Factors
Pertains to aspects of society and social behavior that influence individuals' lives, including culture, ethnicity, social class, and group dynamics.
Risk Assessment
The process of identifying, analyzing, and evaluating the potential risks involved in a specific situation or for a specific entity.
Developmental Stages
represent the phases of growth and development that individuals pass through from infancy to adulthood, encompassing physical, cognitive, and emotional changes.
Q12: Elementary steps in a reaction mechanism often
Q22: If an octahedral iron(II)complex is paramagnetic,which
Q29: As bound ligands,which of the following causes
Q30: Which of the following statements concerning
Q42: What mass of MgO would be produced
Q47: Which of the following species have geometric
Q51: Starch and cellulose are polysaccharides made up
Q61: What is the conjugate base of [Fe(H<sub>2</sub>O)<sub>6</sub>]<sup>3+</sup>(aq)?<br>A)
Q64: The standard free energy change associated
Q76: A buffer is composed of 0.400 mol