In an octahedral complex,electrons in 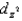 and
and 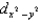
Orbitals experience a greater repulsion from the lone pairs of electrons on ligands because these orbitals
Definitions:
Reinforces
Stimuli that strengthen or increase the likelihood of a behavior by being presented as a consequence of that behavior.
Calf
A young bovine animal, especially the young of the domestic cow or a young elephant, whale, hippo, or moose.
Response Class
A group of behaviors that serve the same function or produce the same outcome, even though they may look different.
Teacher's Attention
The focus or notice that a teacher gives to their students, which can serve as a form of reinforcement in the classroom.
Q3: Which element has the highest density?<br>A) Zn<br>B)
Q9: What is the pH of the
Q18: A business firm may have to comply
Q30: In making business decisions, Glenda, a personnel
Q39: Which of the following statements concerning enzyme
Q51: Calculate <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg" alt=" Calculate
Q53: Which of the following chemical equations
Q55: What is the equilibrium constant for
Q64: Edie files a suit against Frank.If this
Q66: Formulas for derivatives of hydrocarbons may be