The temperature at which the following process reaches equilibrium at 1.0 atm is the normal boiling point of hydrogen peroxide.
H2O2(l) 
H2O2(g)
Use the following thermodynamic information at 298 K to determine this temperature.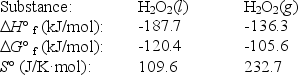
Definitions:
Erroneous Information
Information that is incorrect, misleading, or based on errors.
Salesperson
An individual who sells products or services, often directly to customers, playing a key role in business revenue generation and customer interaction.
Denial
A defense mechanism where an individual refuses to accept reality or facts, often to avoid discomfort.
Readiness to Buy
The stage at which a potential customer is prepared or inclined to make a purchase, influenced by various factors including awareness, interest, and financial capability.
Q3: Examine the following half-reactions and select the
Q7: Fill in missing sub- and superscripts for
Q12: Consider the figure below which shows
Q20: Advantages to borrowing in the private market
Q36: Which of the following is generally used
Q38: You are studying the rate of
Q42: Which of the following statements is true?<br>A)A
Q57: Octahedral complexes can exhibit geometric,optical,and linkage isomerism.
Q62: Consider the following octahedral complex structures,each involving
Q100: Given the capital asset pricing model,a security