Diatomic O2 can react with the element magnesium to form magnesium oxide (MgO) .The balanced chemical equation is: 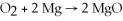
If 4 moles of magnesium totally reacted with more than enough O2,how many moles of MgO would be expected to form?
Definitions:
Hypnosis Sessions
Sessions conducted by a trained therapist in which a person experiences deep relaxation and heightened focus, often used for therapeutic purposes.
REM Sleep Increase
A phase in sleep characterized by rapid eye movement, where dreaming occurs and the body increases its brain activity.
Stage 3 and 4 Sleep
The deepest phases of non-REM sleep, crucial for physical restoration and health, involving further decrease in brain and body activity compared to earlier stages.
Sleep Patterns
The cyclical patterns of sleep, including stages of REM and non-REM sleep, that repeat through the night.
Q4: What are the coefficients for the following
Q7: Which among the following statements is TRUE?<br>A)The
Q15: Light is a type of matter.
Q24: What is the gas produced when hydrochloric
Q29: Which compound listed below will dissolve in
Q42: The correct electron configuration for fluorine is:
Q47: Stoichiometry is a chemist's version of following
Q50: The atomic number of nitrogen is 14.01.
Q62: The names of the elements whose symbols
Q89: What is the theoretical yield in grams