Multiple Choice
What is the balanced oxidation half-reaction for the following unbalanced redox reaction? 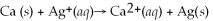
Definitions:
Related Questions
Q2: The burning of fossil fuels produces nitrogen
Q3: The oxygen in the air we breath
Q10: A large equilibrium constant <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6109/.jpg" alt="A
Q14: Which base is found in RNA but
Q28: Amino acids contain a(n):<br>A)amine group.<br>B)carboxylic acid group.<br>C)"R"
Q59: The disulfide bond between two cysteine molecules<br>A)is
Q75: For the reaction 2 <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6109/.jpg" alt="For
Q87: A pH of 7 is equivalent to
Q98: Which solution below would be considered the
Q114: H⁺ is called the hydronium ion.