Calculate the 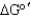 of a redox reaction of pyruvate and hydrogen under standard conditions using the table below.
of a redox reaction of pyruvate and hydrogen under standard conditions using the table below. 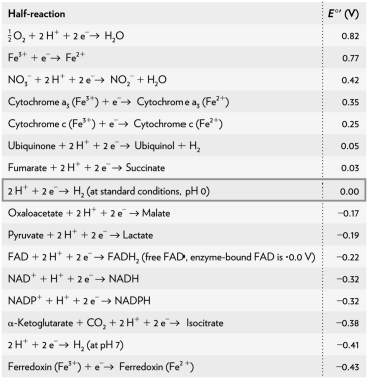
Definitions:
Free Will
The concept that humans have the ability to make choices that are not predetermined by past events or natural laws.
Funder's First Law
The psychological principle stating that "Great strengths are usually great weaknesses, and surprisingly often the opposite is true as well," highlighting the complexity of personality traits.
Psychological Triad
Refers to the three components of psychological analysis: thoughts, feelings, and behaviors.
Pigeonholing
The practice of classifying or categorizing people, ideas, or objects narrowly and often unfairly, limiting potential or understanding.
Q11: Below is the molecular structure of a
Q26: The conversion of L-proline to D-proline
Q43: What is the purpose of the first
Q46: Below is shown the structure of a
Q53: What enzyme uses the proton motive force
Q58: How is penicillin inactivated by penicillin-resistant bacteria?<br>A)
Q63: Consider a system where a passive transport
Q69: Brown adipose tissue is brown in color
Q85: What is the driving force for ATP
Q86: Submitochondrial particles are inside-out pieces of the