The reaction of methane with water to form carbon dioxide and hydrogen is non-spontaneous at 298 K. At what temperature will this system make the transition from non-spontaneous to spontaneous? The data refer to 298 K. 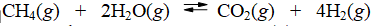
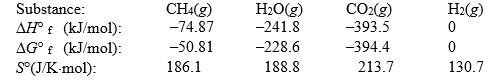
Definitions:
Athletes
Individuals who engage in physical sports, exercises, or games, requiring skill, physical agility, and often competitive nature.
Role Clarity
The extent to which one's job responsibilities, expectations, and boundaries are clearly understood.
Role Acceptance
The process by which individuals agree to, and start performing, the behaviors expected of them in a particular role within a group or organization.
Role Performance
The enactment of behaviors or actions associated with a particular role in society or a specific context.
Q2: The substance Ba(OH)<sub>2 </sub>is considered<br>A) a weak
Q25: A 25.0-mL sample of 1.00 M NH<sub>3
Q25: The reaction of nitrogen with oxygen to
Q39: A secondary cell (battery) can operate either
Q41: For all processes, both q and <font
Q45: Nitric oxide and bromine were allowed to
Q52: Calcium hydroxide, which reacts with carbon dioxide
Q57: What is the [H<sub>3</sub>O<sup>+</sup>] in a solution
Q83: Hydrofluoric acid (HF) has a K<sub>a</sub> value
Q92: In complexes of transition metals, the maximum