Multiple Choice
The temperature at which the following process reaches equilibrium at 1.0 atm is the normal melting point for phosphoric acid. 
Use the following thermodynamic data at 298 K to determine this temperature. 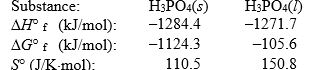
Definitions:
Related Questions
Q9: What will be the effect of adding
Q12: (a) Write a balanced equation representing the
Q17: The crystal field splitting energy, <font face="symbol"></font>,<br>A)
Q20: The formation constant for the reaction <br><img
Q22: Explain how the number of protons and
Q25: Which one of the following statements about
Q33: Propanoic acid (CH<sub>3</sub>CH<sub>2</sub>COOH) has a K<sub>a</sub> of
Q57: Manganese dioxide (MnO<sub>2</sub>) for use in dry
Q66: Nitric oxide is formed in automobile exhaust
Q71: What is the pH of a buffer