Calculate E°cell and indicate whether the overall reaction shown is spontaneous or nonspontaneous. 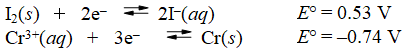
reaction: 2Cr(s) + 3I2(s) 2Cr3+(aq) + (aq) + 6I-(aq)
Definitions:
Consumer Choice
The range of preferences and decisions consumers face regarding the use of products, influenced by income, price, tastes, and preferences.
Demand for Product
The quantity of a good or service that consumers are willing and able to purchase at various prices during a specified period.
Budget Constraint
An economic framework representing the combinations of goods and services an individual can purchase, given their income and the prices of those goods and services.
Indifference Curve
A graphical representation showing combinations of goods between which a consumer is indifferent, implying equal utility.
Q7: In the gas phase at 500.°C, cyclopropane
Q13: Calculate the <font face="symbol"></font>H°<sub>rxn</sub> for the following
Q31: The purpose of anodizing aluminum is to
Q39: A solution is prepared by adding 0.10
Q59: All weak acids have strong conjugate bases.
Q87: The end point in a titration is
Q91: Hydrogen sulfide can be formed in the
Q92: The value of the equilibrium constant for
Q95: A concentration cell consists of two Al/Al<sup>3+</sup>electrodes.
Q112: At the equivalence point in an acid-base