Calculate E°cell and indicate whether the overall reaction shown is spontaneous or nonspontaneous. 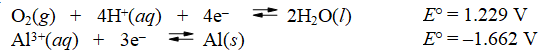
Overall reaction: 4Al(s) + 3O2(g) + 12H+(aq) 4Al3+(aq) + 6H2O(l)
Definitions:
Alcohol
A psychoactive substance found in beverages such as beer, wine, and spirits, known for its effects on the central nervous system.
Depressants
A category of drugs that reduce neural activity and slow down the functions of the brain and body.
Psychoactive Drugs
Substances that change brain function, resulting in alterations in perception, mood, consciousness, cognition, or behavior.
Stimulants
Psychoactive drugs, including caffeine, nicotine, amphetamines, and cocaine, that increase the central nervous system’s activity.
Q11: Blood sample collection from a peripheral vascular
Q19: A certain process has <font face="symbol"></font>H° >
Q24: The temperature at which the following process
Q33: An ideal gas (the system) is contained
Q34: Radioactive decay follows zero-order kinetics.
Q38: The _ process uses a boiling 30%
Q54: Write the mass-action expression, Q<sub>c </sub>, for
Q58: What is the final temperature when 20.0
Q71: Which of the following solids is commonly
Q78: The value of E°<sub>cell</sub> for the reaction