Refer to the figure. 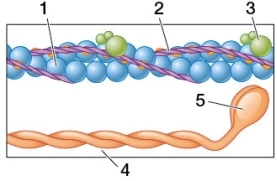 If the purple structure labeled 2 was not present, what would be the most likely position for the structure labeled 5?
If the purple structure labeled 2 was not present, what would be the most likely position for the structure labeled 5?
Definitions:
σ Bonds
The strongest type of covalent chemical bond formed from the head-on overlapping of atomic orbitals, which allows for the free rotation of the bonded atoms around the bond axis.
σ Molecular Orbital
A type of molecular orbital formed by the head-on overlapping of atomic orbitals, characterized by electron density along the axis connecting the nuclei of the atoms involved.
Nodes
Points along a standing wave where the wave has minimal amplitude. In quantum mechanics, it refers to points where the probability density of a particle is zero.
Carbon-Carbon
Describes bonds or interactions specifically between carbon atoms within molecules, crucial for organic chemistry.
Q2: A high _ will lead to an
Q2: Which statement about insect muscles is true?<br>A)
Q5: All of the following characterize taste buds
Q55: Which of the following is not true
Q93: The ascending pathways of the reticular activating
Q126: The division of the autonomic nervous system
Q134: In vertebrate embryos, the brain initially has
Q176: Which feature about sarcomeres is true?<br>A) The
Q253: The receptive field of a retinal ganglion
Q255: Refer to the figure. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg" alt="Refer