The figure shows a pV diagram for 4.3 g of oxygen gas (O2) in a sealed container. The temperature T1 of the gas in state 1 is 21°C. What are the temperatures T3 and T4 of the gas in states 3 and 4? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K, and the ATOMIC weight of oxygen is 16 g/mol. 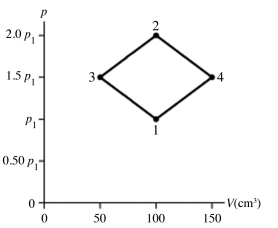
Definitions:
Lung Cancer Deaths
Fatal outcomes resulting from lung cancer, a type of cancer that begins in the lungs and is often associated with smoking.
Negative Reinforcing
A behavior modification process where the removal of an unpleasant stimulus strengthens a behavior.
Negative Reinforcer
A stimulus that, when removed after a behavior occurs, increases the likelihood of that behavior being repeated.
Reduction In Tension
A process or action that decreases stress or anxiety levels in individuals or groups.
Q6: A round loop of diameter 12 cm,
Q11: A -7.0-μC point charge has a positively
Q20: A fixed amount of ideal gas goes
Q20: A boy on a bicycle approaches a
Q21: A negative charge, if free, will tend
Q25: A proton is first accelerated from rest
Q37: A charge Q is uniformly spread over
Q40: An ideal monatomic gas cools from 455.0
Q62: You are driving along a highway at
Q66: A pipe that is 120 cm long