A certain diatomic molecule emits a photon of energy 1.20 eV when it makes a transition from the n = 1 vibrational state to the next lower vibrational state. If the molecule made a transition from the n = 2 state to the n = 1 state, what would be the energy of the photon it would emit? (h = 6.626 × 10-34 J ∙ s, 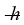 = 1.055 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
= 1.055 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
Definitions:
Commonalities
Features or characteristics that are shared by different groups or things.
Cultural Value
The shared beliefs, norms, and practices that shape the social behaviors and expectations within a specific group or society.
Individualism
A social philosophy advocating for individual autonomy over the control of the collective or government.
Social Framework
The structure of societal norms, values, and institutions that shapes the interactions and behaviors of groups and individuals within a community.
Q7: Which of the following has the greatest
Q20: A vibrating diatomic molecule in its ground
Q20: Light strikes a 5.0-cm thick sheet of
Q38: Death-related practices among Hispanic Americans often involve<br>A)
Q41: An alkali metal atom is in the
Q62: A fisherman in a stream 39 cm
Q64: A certain nucleus containing 8 protons and
Q66: Which of the following statements about β<sup>+</sup>
Q67: The focal length of a thin lens
Q83: The maximum permissible workday dose for occupational