Use the following to answer the question: 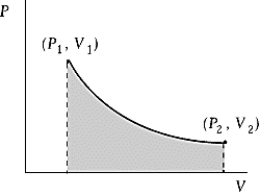
-An ideal gas initially at 100ºC and pressure P1 = 250 kPa occupies a volume V1 = 4.5 L.It undergoes a quasistatic,isothermal expansion until its pressure is reduced to 150 kPa.How much does the internal energy of the gas change during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
Definitions:
In-progress Inventory
Items that are currently being manufactured or are in the process of being transformed from raw materials to finished goods.
Finished Goods Inventory
An end item ready to be sold, but still an asset on the company’s books.
Raw Materials
Basic materials that are processed or refined to create a final product in manufacturing.
Monthly Sales
The total revenue or number of units sold by a business during a calendar month.
Q24: While you are standing on a corner,a
Q26: A particle moving in simple harmonic motion
Q33: The electrical potential 2.5 cm from a
Q38: Which diagram best represents the electric field
Q47: The equipartition theorem<br>A)fails to explain the fact
Q49: A solid spherical conductor of radius 15
Q59: An ideal gas initially at 50ºC and
Q69: A rocket ship is propelled vertically up
Q72: Which of the following is an assumption
Q73: A hurricane strength wind is blowing past