Use the following diagram to answer the next problem. 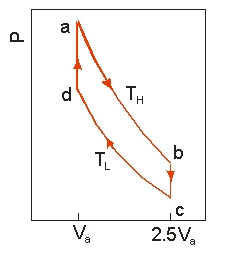 An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at
An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at
TH = 400 K and cold reservoir at TL = 300 K, in a cycle from a→b→c→d→a. From a→b the gas undergoes an isothermal expansion, changing its volume from Va to 2.5Va. From b→c, the pressure is reduced at a constant volume. From c→d, the gas undergoes an isothermal compression, and from d→a, the pressure is increased at a constant volume until the gas is back at the original condition at a.
-If the engine operates at 50 cycles per second,the power output is
Definitions:
Total Assets
The sum of all current and non-current assets owned by a business.
Owner's Equity Statement
A financial document showing the changes in the equity of a business entity over a period, including investments, withdrawals, and net income or loss.
Sole Proprietorship
A business owned by a single individual who is responsible for its liabilities and entitled to its profits.
Proprietorship
A business structure owned by a single individual, where the owner and the business are legally treated as the same.
Q7: At what Kelvin temperature does the
Q7: The efficiency of a Carnot engine operating
Q45: If you were to quadruple the speed
Q54: Earth receives approximately 1.7 <span
Q56: A thermodynamic system is taken in equilibrium
Q76: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6080/.jpg" alt=" The temperature of
Q80: Normal human body temperature is 98.6ºF. What
Q87: What is the major product of the
Q89: The solution to the differential equation
Q100: The pressure of a mass of