Multiple Choice
For the following acid/base reaction which statement is true taking S into consideration? 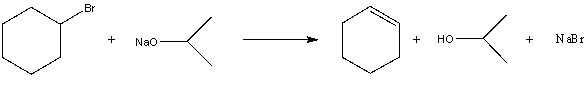
Definitions:
Related Questions
Q5: Write an equation that shows the reaction
Q30: The compound below is an adrenocortical
Q47: Hydrogen atom(s)from which position(s)is (are)most likely to
Q91: The reaction, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5902/.jpg" alt=" The
Q95: It is impossible to have pK<sub>a</sub> values
Q99: Which of these is the weakest of
Q122: An unknown compound,A,has the molecular formula C<sub>7</sub>H<sub>12</sub>.On
Q130: Which of the following is bicyclo[3.2.2]nonane? <img
Q146: For the following reaction sequence (it is
Q156: When the 1s orbitals of two hydrogen