The reaction, 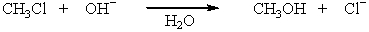 has the following thermodynamic values at 27.0 ºC: H = -75.3 kJ mol-1; S = 54.4 J K-1 mol-1.What is the value of G for this reaction?
has the following thermodynamic values at 27.0 ºC: H = -75.3 kJ mol-1; S = 54.4 J K-1 mol-1.What is the value of G for this reaction?
Definitions:
BCG Matrix
A strategic business tool devised by the Boston Consulting Group to help organizations analyze their portfolio of products or services based on market growth and market share.
SBUs
Strategic Business Units are individual units within a larger company that operate as separate businesses, each with its own mission, products, and market.
Portfolio
A collection of investments held by an individual or institution, which could include stocks, bonds, real estate, and other financial assets.
BCG Matrix
A framework that categorizes a company's products or business units into four types (stars, cash cows, question marks, dogs) based on market growth and market share, guiding resource allocation decisions.
Q2: Typically,increasing the concentration of the nucleophile of
Q6: Which reaction of these potential acids and
Q36: The hybridization state of the charged carbon
Q41: Mono-bromination of the following alkane, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5902/.jpg"
Q53: Which of the following is a Lewis
Q138: What is the structure of the compound
Q153: Consider the expected splitting of signal "b"
Q158: Draw a dash-wedge structure for (3S,4R)-4-chloro-3,5-dimethylhex-1-yne
Q193: What is the major product for the
Q204: When diastereomers I and II undergo an