The reaction, 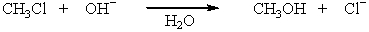 has the following thermodynamic values at 27.0 ºC: H = -75.3 kJ mol-1; S = 54.4 J K-1 mol-1.What is the value of G for this reaction?
has the following thermodynamic values at 27.0 ºC: H = -75.3 kJ mol-1; S = 54.4 J K-1 mol-1.What is the value of G for this reaction?
Definitions:
Organizational Design
The process of shaping an organization's structure and roles to align with its objectives, strategy, and culture.
Structural Elements
The fundamental components that constitute the framework of a structure, such as buildings, bridges, or sentences in linguistics.
Firm's Competitor
An organization or entity that operates in the same or similar industry and offers a comparable product or service.
Long-Linked Technology
Technology that involves sequential processes and operations across different stages of production or service delivery.
Q2: Select the structure of the major product
Q52: Draw the structures of all stereoisomers of
Q55: Increasing the concentration of either of the
Q73: Predict the <sup>1</sup>H NMR spectrum of cis-1,4-cyclohexanediol
Q82: Which of the following is an
Q105: A molecule must possess at least one
Q117: CH<sub>3</sub>CHBrCH<sub>2</sub>CHClCH<sub>3</sub> is the generalized representation of what
Q138: What is the structure of the compound
Q138: Draw a dash-wedge structure for (2R,4S)-2,4-dibromo-2-chloropentane
Q142: What is the major product for the