The specific heat of copper is 0.0920 cal/g °C,and the specific heat of silver is 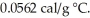 If 100 cal of heat is added to one g of each metal at 25 °C,what is the expected result?
If 100 cal of heat is added to one g of each metal at 25 °C,what is the expected result?
Definitions:
Utilitarianism
An ethical theory that posits the best action is the one that maximizes utility, usually defined as that which produces the greatest well-being of the greatest number of people.
Ethical Implications
The moral consequences or considerations of a decision, action, or policy in societal and individual terms.
Proprietary Information
Information that is owned by an individual or company, regarded as a trade secret or confidential knowledge.
Declaration of Helsinki
An international set of ethical principles for medical research involving human subjects, developed by the World Medical Association.
Q5: The codon is found on _,and the
Q16: Which of the following polyatomic ions has
Q33: Which of the following examples illustrates a
Q36: -15 °C
Q56: A barometer is usually filled with _.
Q69: The mass number of an atom can
Q73: Is the following reaction exothermic or endothermic?<br>2C2H6
Q77: The atomic number of sodium is 23.
Q81: The following is a decomposition reaction.<br>2KCl <img
Q88: The nuclear reaction shown below is an