Using the following data,determine the standard cell potential 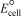 for the electrochemical cell constructed based on the following unbalanced reaction expression: Al(s) + Cr3+(aq) Al3+(g) + Cr2+(aq) .
for the electrochemical cell constructed based on the following unbalanced reaction expression: Al(s) + Cr3+(aq) Al3+(g) + Cr2+(aq) .
Half-reaction Standard reduction potential
Al3+(aq) + 3 e- Al(s) -1.66
Cr3+(aq) + e- Cr2+(aq) -0.41
Definitions:
Negative Freedom
The concept of freedom defined by the absence of constraints or interference from others, emphasizing what individuals are free from.
Sufficient
Adequate to meet the needs of a situation or a proposed end without needing more.
Sartre
Jean-Paul Sartre, a French existentialist philosopher, playwright, novelist, and political activist known for his explorations of freedom and existentialism.
Extreme Circumstances
Situations that are highly unusual or exceptional, often involving severe stress or urgency.
Q12: Based on the information in the
Q24: Compare the packing efficiency of face-centered cubic
Q27: Because of recent advances in recovery technology,the
Q75: Which of the following is/are true regarding
Q93: Using the following data,determine the standard
Q108: Which of the following releases the most
Q133: Estimate the value of K<sub>f</sub> for
Q143: The unit of electrical power,watt (W),is defined
Q157: The molar solubility,s,for Al(OH)<sub>3</sub> is written as
Q214: Briefly explain why the relative strengths of